Based-Sorbitol Electrolyte Additive for Reversible Zn Electrochemistry
Authors
Qiong Sun, Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Renewable Energy Conversion and Storage Center (RECAST), College of Chemistry, Nankai University, Tianjin, 300071, China
Hai-Hui Du, Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Renewable Energy Conversion and Storage Center (RECAST), College of Chemistry, Nankai University, Tianjin, 300071, China
Tian-Jiang Sun, Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Renewable Energy Conversion and Storage Center (RECAST), College of Chemistry, Nankai University, Tianjin, 300071, China
Dian-Tao Li, Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Renewable Energy Conversion and Storage Center (RECAST), College of Chemistry, Nankai University, Tianjin, 300071, China
Min Cheng, Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Renewable Energy Conversion and Storage Center (RECAST), College of Chemistry, Nankai University, Tianjin, 300071, China
Jing Liang, Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Renewable Energy Conversion and Storage Center (RECAST), College of Chemistry, Nankai University, Tianjin, 300071, ChinaFollow
Hai-Xia Li, Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Renewable Energy Conversion and Storage Center (RECAST), College of Chemistry, Nankai University, Tianjin, 300071, ChinaFollow
Zhan-Liang Tao, Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Renewable Energy Conversion and Storage Center (RECAST), College of Chemistry, Nankai University, Tianjin, 300071, China
Corresponding Author(s)
Haixia Li(lihaixia@nankai.edu.cn);
Jing Liang(liangjing@nankai.edu.cn)
Abstract
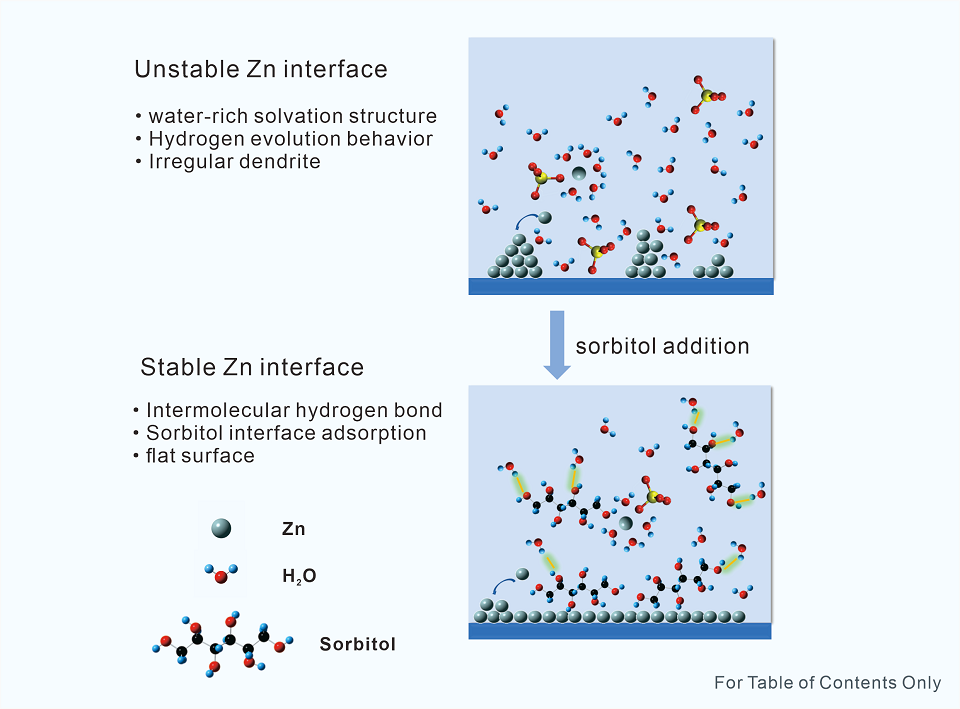
The unstable Zn interfaces caused by undesired dendrite and parasitic side reactions greatly hinder the development of aqueous Zn ion batteries. Herein, hydroxy-rich sorbitol was used as an additive to reshape the solvation structure and modulate the zinc interface chemistry. The strong interaction between sorbitol and both water molecules and Zn electrode can reduce the free water activity, optimize the solvation shell of water and Zn2+ ions, regulate the formation of local H2O-poor environment on the surface of Zn electrode, and effectively inhibit the decomposition of water molecules, thus the thermodynamically stable and highly reversible Zn electrochemistry is obtained. As a result, Zn/Zn symmetric cells with the sorbitol additive realized an excellent cycling life of 2000 h under 1 mA·cm-2 and 1 mAh·cm-2 and over 250 h under 5 mA·cm-2 and 5 mAh·cm-2. Moreover, the Zn/Cu asymmetric cells with the sorbitol additive achieved a high Coulomb efficiency of 99.6%, obtain elevated performance than that with pure 2 M ZnSO4 electrolyte. And the constructed Zn/PNDA batteries can be stably discharged for 2300 cycles at 1 A·g-1 with an excellent capacity retention rate. This result indicates that based-sorbitol addictive electrolytes under 1 M sorbitol successfully elevated reversible zinc electrochemistry.
Graphical Abstract
Keywords
aqueous zinc ion batteries, dendrite, sorbitol additive, solvation regulation, interface modulation
DOI
10.61558/2993-074X.3447
Recommended Citation
Qiong Sun, Hai-Hui Du, Tian-Jiang Sun, Dian-Tao Li, Min Cheng, Jing Liang, Hai-Xia Li, Zhan-Liang Tao. Based-Sorbitol Electrolyte Additive for Reversible Zn Electrochemistry[J]. Journal of Electrochemistry, doi: 10.61558/2993-074X.3447.

