Authors
Meng-yan HOU, Department of Chemistry, Fudan University, Shanghai, 200433, China;Shanghai Institute of Applied Physics, CAS, Shanghai, 201800;
Hong-liang BAO, Shanghai Institute of Applied Physics, CAS, Shanghai, 201800;
Ke WANG, Department of Chemistry, Fudan University, Shanghai, 200433, China;
Jian-qiang WANG, Shanghai Institute of Applied Physics, CAS, Shanghai, 201800;Follow
Yong-yao XIA, Department of Chemistry, Fudan University, Shanghai, 200433, China;Follow
Corresponding Author
Jian-qiang WANG(wangjianqiang@sinap.ac.cn);
Yong-yao XIA(yyxia@fudan.edu.cn)
Abstract
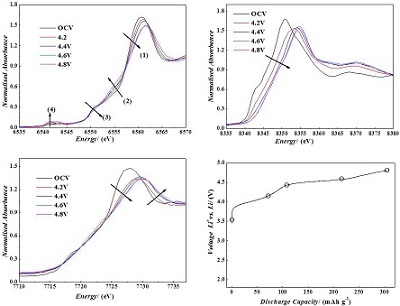
A series of the lithium-rich and manganese-based layered structure xLi2MnO3•(1-x)LiMn1/3Ni1/3Co1/3O2 (x = 0.3,0.5,0.7) materials were synthesized by a co-precipitation method, and followed by a solid-state reaction process. By comparing the first cycle efficiency, the reversible discharge capacity, the cycling stability and the voltage decay during the charge/discharge cycling process, the material with the composition of 0.5Li2MnO3•0.5LiMn1/3Ni1/3Co1/3O2was found to show the best electrochemical performance. The lithium storage mechanism and thermal stability of the de-lithiated compound were also investigated by in situ X-ray absorption fine structure (XAFS) spectroscopy and differential scanning calorimetry (DSC) techniques. The results of XAFS indicates that during the charging process to 4.5 V, the Ni and Co ions are oxidized to Ni4+ and Co4+, respectively, while the Mn ion remains Mn4+.
Graphical Abstract
Keywords
lithium-rich layered oxides, cathode material, lithium-ion battery, in situ X-ray absorption fine structure
Publication Date
2016-06-28
Online Available Date
2016-06-02
Recommended Citation
Meng-yan HOU, Hong-liang BAO, Ke WANG, Jian-qiang WANG, Yong-yao XIA.
Electrochemical and in situ X-ray Absorption Fine Structure Study of Li-Rich Cathode Materials[J]. Journal of Electrochemistry,
2016
,
22(3): 288-298.
DOI: 10.13208/j.electrochem.151248
Available at:
https://jelectrochem.xmu.edu.cn/journal/vol22/iss3/8
References
[1] Ohzuku T, Makimura Y. Layered lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for lithium-ion batteries[J]. Chemistry Letters, 2001, 7: 642-643.
[2] Chen C H, Liu J, Stoll M E, et al. Aluminum-doped lithium nickel cobalt oxide electrodes for high-power lithium-ion batteries [J]. Journal of Power Sources, 2004, 128(2): 278-285.
[3] Thackeray M M, Kang S H, Johnson C S, et al. Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries[J]. Journal of Materials Chemistry, 2007, 17(30): 3112-3125.
[4] Kim J S, Johnson C S, Thackeray M M. Layered xLiMO2·(1-x)Li2M'O3 electrodes for lithium batteries: A study of 0.95LiMn0.5Ni0.5O2·0.05Li2TiO3[J]. Electrochemistry Communications, 2002, 4(3): 205-209.
[5] Kim J S, Johnson C S, Vaughey J T, et al. Electrochemical and structural properties of xLi2M'O3·(1-x)LiMn0.5Ni0.5O2 eIectrodes for lithium batteries (M' = Ti, Mn, Zr; 0 ¤ x ¤ 0.3)[J]. Chemistry of Materials, 2004, 16(10): 1996-2006.
[6] Johnson C S, Li N C, Lefief C, et al. Synthesis, characterization and electrochemistry of lithium battery electrodes: xLi2MnO3·(1-x)LiMn0.333Ni0.333Co0.333O2 (0 ¤ x ¤ 0.7)[J]. Chemistry of Materials, 2008, 20(19): 6095-6106.
[7] Amalraj F., Kovacheva D, Talianker M, et al. Synthesis of integrated cathode materials xLi2MnO3·(1-x)LiMn1/3Ni1/3Co1/3O2 (x = 0.3, 0.5, 0.7) and studies of their electrochemical behavior (vol 157, A1121, 2010) [J]. Journal of the Electrochemical Society, 2010, 157(11): S19-S19.
[8] Toprakci O, Toprakci H A K, Li Y, et al. Synthesis and characterization of xLi2MnO3·(1-x)LiMn1/3Ni1/3Co1/3O2 composite cathode materials for rechargeable lithium-ion batteries [J]. Journal of Power Sources, 2013, 241: 522-528.
[9] Zhao Y J, Ren W F, Wu R, et al. Improved molten salt synthesis and structure evolution upon cycling of 0.5Li2MnO3·0.5LiCoO2 in lithium-ion batteries[J]. Journal of Solid State Electrochemistry, 2013, 17(8): 2259-2267.
[10] Li J G, Wang L, Wang L, et al. Synthesis and characterization of Li(Li0.23Mn0.47Fe0.2Ni0.1)O2 cathode material for Li-ion batteries[J]. Journal of Power Sources, 2013, 244: 652-657.
[11] Ohzuku T, Nagayama M, Tsuji K, et al. High-capacity lithium insertion materials of lithium nickel manganese oxides for advanced lithium-ion batteries: Toward rechargeable capacity more than 300 mAh·g-1[J]. Journal of Materials Chemistry, 2011, 21(27): 10179-10188.
[12] Gong Z L, Liu H S, Guo X J, et al. Effects of preparation methods of LiNi0.8Co0.2O2 cathode materials on their morphology and electrochemical performance[J]. Journal of Power Sources, 2004, 136(1): 139-144.
[13] Yoon W S, Iannopollo S, Grey C P, et al. Local structure and cation ordering in O3 lithium nickel manganese oxides with stoichiometry LiNixMn(2-x)/3Li(1-2x)/3O2 - NMR studies and first principles calculations[J]. Electrochemical and Solid State Letters, 2004, 7(7): A167-A171.
[14] Kim G Y, Yi S B, Park Y J, et al. Electrochemical behaviors of Li Li(1-x)/3Mn(2-x)/3Nix/3Cox/3 O2 cathode series (0 < x < 1) synthesized by sucrose combustion process for high capacity lithium ion batteries [J]. Materials Research Bulletin, 2008, 43(12): 3543-3552.
[15] Lee E S, Huq A, Manthiram A. Understanding the effect of synthesis temperature on the structural and electrochemical characteristics of layered-spinel composite cathodes for lithium-ion batteries [J]. Journal of Power Sources, 2013, 240: 193-203.
[16] Liu J L, Liu J Y, Wang R H, et al. Degradation and structural evolution of xLi2MnO3·(1-x)LiMn1/3Ni1/3Co1/3O2 during cycling[J]. Journal of the Electrochemical Society, 2014, 161(1): A160-A167.
[17] Deng H, Belharouak I, Wu H, et al. Effect of cobalt incorporation and lithium enrichment in lithium nickel manganese oxides[J]. Journal of the Electrochemical Society, 2010, 157(7): A776-A781.
[18] Li Z, Chernova N A, Feng J J, et al. Stability and rate capability of Al substituted lithium-rich high-manganese content oxide materials for Li-ion batteries[J]. Journal of the Electrochemical Society, 2012, 159(2): A116-A120.
[19] Rana J, Kloepsch R, Li J, et al. On the structural integrity and electrochemical activity of a 0.5Li2MnO3center dot 0.5LiCoO2 cathode material for lithium-ion batteries[J]. Journal of Materials Chemistry A, 2014, 2(24): 9099-9110.
[20] Croy J R, Kang S H, Balasubramanian M, et al. Li2MnO3-based composite cathodes for lithium batteries: A novel synthesis approach and new structures[J]. Electrochemistry Communications, 2011, 13(10): 1063-1066.
[21] Ito A, Sato Y, Sanada T, et al. In situ X-ray absorption spectroscopic study of Li-rich layered cathode material LiNi0.17Li0.2Co0.07Mn0.56O2[J]. Journal of Power Sources, 2011, 196(16): 6828-6834.
[22] Balasubramanian M, McBreen J, Davidson I J, et al. In situ X-ray absorption study of a layered manganese-chromium oxide-based cathode material[J]. Journal of the Electrochemical Society, 2002, 149(2): A176-A184.

