Authors
Ya-ning HE, School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, Huanan,China;
Liang YUAN, School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, Huanan,China;
Zhi-ying DING, School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, Huanan,China;
Shi-jun LIU, School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, Huanan,China;Follow
Corresponding Author
Shi-jun LIU(shijunliu@csu.edu.cn)
Abstract
The effects of allylthiourea (ATU) concentration on the cathodic polarization behaviour, nucleation and surface morphology of nickel electrodeposited on the glassy carbon electrode from ammonia-ammonium chloride-water (NH3-NH4Cl-H2O) solutions were investigated by cyclic voltammogry, cathodic polarization and current transient methods. The results revealed that the addition of ATU inhibited nickel deposition, which was enhanced with an increase in ATU concentration from 5 to 50 mg•L-1. The initial deposition kinetics corresponded to a model including instantaneous nucleation and diffusion controlled growth. In the presence of ATU, the initial nucleation of nickel electrocrystallisation remained unchanged. However, the number density of nuclei increased and the crystal growth rate decreased. Furthermore, the addition of ATU apparently made the grains finer, leading to the formation of a more compact and uniform nickel deposit as compared with that without ATU.
Graphical Abstract
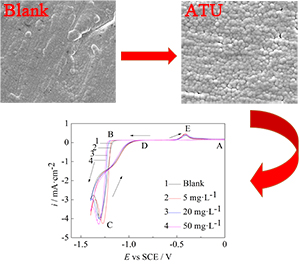
Keywords
nickel electrodeposition, NH3-NH4Cl-H2O solution, allyl thiourea, additive
Publication Date
2017-12-28
Online Available Date
2017-02-13
Recommended Citation
Ya-ning HE, Liang YUAN, Zhi-ying DING, Shi-jun LIU.
Effect of allyl thiourea on nickel electrodeposition from solution containing ammonia and chloride[J]. Journal of Electrochemistry,
2017
,
23(6): 638-644.
DOI: 10.13208/j.electrochem.161217
Available at:
https://jelectrochem.xmu.edu.cn/journal/vol23/iss6/3
References
[1] Mackenzie M, Virnig M, Feather A. The recovery of nickel from high-pressure acid leach solutions using mixed hydroxide product LIX ® 84-INS technology[J]. Minerals Engineering,2006,19(12):1220-1233.
[2] Cao H Z(曹华珍), Zheng G Q(郑国渠), Zhi B(支波)et al. Cathodic process of zinc electrowinning in solution containing a mmonia complex[J].Transactions of Nonferrous Metals society of China(有色金属学报),2005,15(4):655-660.
[3] Zheng G Q(郑国渠), Zheng L F(郑利峰)Cao H Z(曹华珍),et al. Nickel electrodeposition from leaching solution containing ammonia and chloride[J]. Transactions of Nonferrous Metals society of China(有色金属学报), 2003,13(1):217-220.
[4] Johnson G R, Turner D R. The Effect of Some Addition Agents on the Kinetics of Copper Electrodeposition from a Sulfate Solution II . Rotating Disk Electrode Experiments[J]. New Zealand Journal of Agricultural Research,1962,109(10):918-922.
[5] Suarez D F, Olson F A. Nodulation of electrodeposited copper in the presence of thiourea[J]. Journal of Applied Electrochemistry,1992,22(22):1002-1010.
[6] Alodan M A. Confocal Laser Scanning Microscopy, Electrochemistry, and Quartz Crystal Microbalance Studies of Leveling Effects of Thiourea on Copper Deposition[J]. Journal of the Electrochemical Society,1998,145(3):957-963.
[7] Awad M K. Semiempirical investigation of the inhibition efficiency of thiourea derivatives as corrosion inhibitors[J]. Journal of Electroanalytical Chemistry,2004,567(2):219-225.
[8] Haseeb A S M A, Schilardi P L, Bolzan A E, et al. Anodisation of copper in thiourea-containing acid solution : Part II. In situ transversal imaging observations. Kinetics of anodic film growth[J]. Journal of Electroanalytical Chemistry,2001,500(12):543-553.
[9] Oniciu L, Mure?an L. Some fundamental aspects of levelling and brightening in metal electrodeposition[J]. Journal of Applied Electrochemistry,1991,21(7):565-574.
[10] Hoekstra J J, Dan T. The Uptake of Sulfur from Plating Brighteners by Copper and Nickel[J]. Journal of the Electrochemical Society,1964,111(2).
[11] Cao H, Yang D, Zhu S, et al. Preparation, characterization, and electrochemical studies of sulfur-bearing nickel in an ammoniacal electrolyte: the influence of thiourea[J]. Journal of Solid State Electrochemistry,2012,16(9):3115-3122.
[12] Shen C B, Wang S G, Yang H Y. The adsorption stability and inhibition by allyl thiourea of bulk nanocrystalline ingot iron in dilute HCl solution. Appl Surf Sci[J]. Applied Surface Science,2006,253(4):2118-2122.
[13] Upadhyay D N, Yegnaraman V. Effect of thiourea and substituted thioureas on copper underpotential deposition on gold[J]. Materials Chemistry & Physics,2000,62(3):247-253.
[14] Chen G L, Lin H, Lu J H, et al. SERS and EQCM studies on the effect of allyl thiourea on copper dissolution and deposition in aqueous sulfuric acid[J]. Journal of Applied Electrochemistry,2008,38(11):1501-1508.
[15] Tang L N, Wang F P. Electrochemical evaluation of allyl thiourea layers on copper surface[J]. Corrosion Science,2008,50(4):1156-1160.
[16] Fletcher S. Some new formulae applicable to electrochemical nucleation/growth/collision[J]. Electrochimica Acta,1983,28(7):917-923.
[17] Mohanty U S, Tripathy B C, Singh P, et al. Effect of pyridine and its derivatives on the electrodeposition of nickel from aqueous sulfate solutions. Part II: Polarization behaviour[J]. Journal of Applied Electrochemistry,2001,31(9):969-972.
[18] Scharifker B, Hills G. Theoretical and experimental studies of multiple nucleation[J]. Electrochimica Acta,1983,28(7):879-889.
[19] Song Y, Tang J, Hu J, et al. Insights into electrodeposition process of nickel from ammonium chloride media with speciation analysis and in situ synchrotron radiation X-ray imaging[J]. Electrochimica Acta,2016,210:812-820.

