Corresponding Author
Shan-fu LU;Yan XIANG
Abstract
The effect of surface modification modes on proton-over-vanadium ion selectivity was studied by spin-coating chitosan-Phosphotungstic Acid (PWA) as a single or double layer on Nafion membrane surface. To suppress the vanadium ions permeation through the Nafion? membrane in a vanadium redox flow battery (VRFB), the single surface-modified Nafion membrane (Nafion/chitosan-PWA)S and double surface-modified Nafion membrane (Nafion/chitosan-PWA)D demonstrated a 89.9% and 92.7% reduction of vanadium ion permeability in comparison with pristine Nafion, respectively. The (Nafion/chitosan-PWA)D exhibited betterhigher selectivity between proton and vanadium ions than the (Nafion/chitosan-PWA)S at the same layer thickness. Furthermore, the columbic efficiency for the VRFB single cell based on the (Nafion/chitosan-PWA)D at an optimized thickness was 93.5% and the energy efficiency was 80.7% at a charge-discharge current density of 30 mA·cm-2, which wereas higher than the (Nafion/chitosan-PWA)S and pristine Nafion membrane. The modified membranes also possessed adequate chemical stability in the VRFB during charge-discharge cycling measurements.
Graphical Abstract
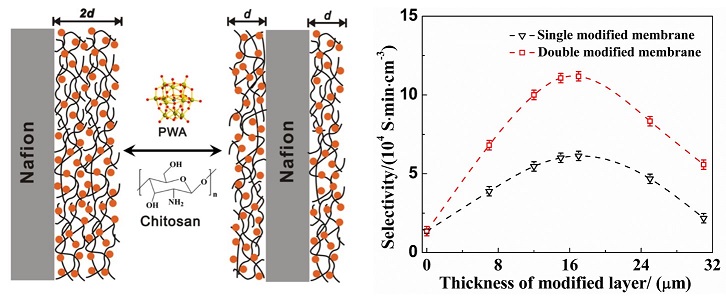
Keywords
Surface modification mode, Vanadium ion permeability, Ionic selectivity, Vanadium redox flow battery
Publication Date
2017-08-25
Online Available Date
2016-04-22
Recommended Citation
Qing-long TAN, Hai-ning WANG, Shan-fu LU, Da-wei LIANG, Chun-xiao WU, Yan XIANG.
Effects of surface modification modes on proton-over-vanadium ion selectivity of Nafion®membrane for application in vanadium redox flow battery[J]. Journal of Electrochemistry,
2017
,
23(4): 160307.
DOI: 10.13208/j.electrochem.160307
Available at:
https://jelectrochem.xmu.edu.cn/journal/vol23/iss4/15
References
[1] Sum E., Rychcik M., Skyllas-Kazacos M. Investigation of the V(V)/V(IV) system for use in the positive half-cell of a redox battery [J]. Journal of Power Sources, 1985, 16(2): 85-95.
[2] Xu Q, Zhao T S, Leung P k. Numerical investigations of flow field designs for vanadium redox flow batteries [J]. Applied Energy, 2013, 105: 47-56.
[3] Zhang C, Zhao T S, Xu Q, et al. Effects of operating temperature on the performance of vanadium redox flow batteries [J]. Applied Energy, 2015, 155: 349-353.
[4] Wang W, Luo Q T, Li B, et al. Recent progress in redox flow battery research and development [J]. Advanced Functional Materials, 2013, 23(8): 970-986.
[5] Shen J, Xi J Y, Zhu W T, et al. A nanocomposite proton exchange membrane based on PVDF, poly (2-acrylamido-2-methyl propylene sulfonic acid), and nano-Al2O3 for direct methanol fuel cells [J]. Journal of Power Sources, 2006, 159(2): 894-899.
[6] Li X F, Zhang H M, Mai Z S, et al. Ion exchange membranes for vanadium redox flow battery (VRB) applications [J]. Energy & Environmental Science, 2011, 4(4): 1147-1160.
[7] Xi J Y, Wu Z H, Qiu X P, et al. Nafion/SiO2 hybrid membrane for vanadium redox flow battery [J]. Journal of Power Sources, 2007, 166(2): 531-536.
[8] Wang Z, Tang H L, Zhang H J, et al. Synthesis of Nafion/CeO2 hybrid for chemically durable proton exchange membrane of fuel cell [J]. Journal of Membrane Science, 2012, 421:201-210.
[9] Teng X G, Zhao Y T, Xi J Y, et al. Nafion/organic silica modified TiO2 composite membrane for vanadium redox flow battery via in situ solgel reactions [J]. Journal of Membrane Science, 2009, 341(1): 149-154.
[10] Teng X G, Zhao Y T, Xi J Y, et al. Nafion/organically modified silicate hybrids membrane for vanadium redox flow battery [J]. Journal of Power Sources, 2009, 189(2): 1240-1246.
[11] Mai Z S, Zhang H M, Li X F, et al. Nafion/polyvinylidene fluoride blend membranes with improved ion selectivity for vanadium redox flow battery application [J]. Journal of Power Sources, 2011, 196(13): 5737-5741.
[12] Jie Z, Tang H L, Mu P. Fabrication and characterization of self-assembled Nafion-SiO2-ePTFE composite membrane of PEM fuel cell [J]. Journal of Membrane Science, 2008, 312(1): 41-47.
[13] Teng X G, Dai J C, Su J, et al. A high performance polytetrafluoroethene/Nafion composite membrane for vanadium redox flow battery application [J]. Journal of Power Sources, 2013, 240:131-139.
[14] Wang N F, Peng S, Lu D, et al. Nafion/TiO2 hybrid membrane fabricated via hydrothermal method for vanadium redox battery [J]. Journal of Solid State Electrochemistry, 2012, 16(4): 1577-1584.
[15] Kim Jihoon, Jeon Jae-Deok, Kwak Seung-Yeop. Nafion-based composite membrane with a permselective layered silicate layer for vanadium redox flow battery [J]. Electrochemistry Communications, 2014, 38:68-70.
[16] Teng X G, Dai J C, Su J, et al. Modification of Nafion membrane using fluorocarbon surfactant for all vanadium redox flow battery [J]. Journal of Membrane Science, 2015, 476: 20-29.
[17] Yang B, Manthiram A. Multilayered membranes with suppressed fuel crossover for direct methanol fuel cells [J]. Electrochemistry communications, 2004, 6(3): 231-236.
[18] Yang M, Lu S F, Lu J L, et al. Layer-by-layer self-assembly of PDDA/PWA-Nafion composite membranes for direct methanol fuel cells [J]. Chemical Communications, 2010, 46(9): 1434-1436.
[19] Mu S C, Tang H L, Wan Z H, et al. Au nanoparticles self-assembled onto Nafion membranes for use as methanol-blocking barriers [J]. Electrochemistry Communications, 2005, 7(11): 1143-1147.
[20] Tang H L, Pan M, Jiang S P, et al. Self-assembling multi-layer Pd nanoparticles onto Nafion¢ membrane to reduce methanol crossover [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2005, 262(1): 65-70.
[21] Xi J Y, Wu Z H, Teng X G, et al. Self-assembled polyelectrolyte multilayer modified Nafion membrane with suppressed vanadium ion crossover for vanadium redox flow batteries [J]. Journal of Materials Chemistry, 2008, 18(11): 1232-1238.
[22] Zeng J, Jiang C P, Wang Y H, et al. Studies on polypyrrole modified nafion membrane for vanadium redox flow battery [J]. Electrochemistry Communications, 2008, 10(3): 372-375.
[23] Luo Q T, Zhang H M, Chen J, et al. Modification of Nafion membrane using interfacial polymerization for vanadium redox flow battery applications [J]. Journal of Membrane Science, 2008, 311(1): 98-103.
[24] Luo Q T, Zhang H M, Chen J, et al. Preparation and characterization of Nafion/SPEEK layered composite membrane and its application in vanadium redox flow battery [J]. Journal of Membrane Science, 2008, 325(2): 553-558.
[25] Xiang Y, Zhang J, Liu Y, et al. Design of an effective methanol-blocking membrane with purple membrane for direct methanol fuel cells [J]. Journal of Membrane Science, 2011, 367(1): 325-331.
[26] Mengatto L, Luna J A, Cabrera M I. Influence of cross-linking density on swelling and estradiol permeation of chitosan membranes [J]. Journal of Materials Science, 2010, 45(4): 1046-1051.
[27] Lu S F, Wu C X, Liang D W, et al. Layer-by-layer self-assembly of Nafion-[CS-PWA] composite membranes with suppressed vanadium ion crossover for vanadium redox flow battery applications [J]. RSC Advances, 2014, 4(47): 24831-24837.
[28] Lu J L, Tang H L, Lu S F, et al. A novel inorganic proton exchange membrane based on self-assembled HPW-meso-silica for direct methanol fuel cells [J]. Journal of Materials Chemistry, 2011, 21(18): 6668-6676.
[29] Jiang S P, Liu Z C, Tian Z Q. Layer-by-Layer Self-Assembly of Composite Polyelectrolyte-Nafion Membranes for Direct Methanol Fuel Cells [J]. Advanced Materials, 2006, 18(8): 1068-1072.
[30] Zhang F X, Zhang H M, Qu C. A Dication Cross-Linked Composite Anion-Exchange Membrane for All-Vanadium Flow Battery Applications [J]. ChemSusChem, 2013, 6(12): 2290-2298.
[31] Pan J, Lu S F, Li Y, et al. High-Performance Alkaline Polymer Electrolyte for Fuel Cell Applications [J]. Advanced Functional Materials, 2010, 20(2): 312-319.
[32] Luo X L, Lu Z Z, Xi J Y, et al. Influences of permeation of vanadium ions through PVDF-g-PSSA membranes on performances of vanadium redox flow batteries [J]. The Journal of Physical Chemistry B, 2005, 109(43): 20310-20314.
[33] Teng X G, Dai J C, Bi FY, et al. Ultra-thin polytetrafluoroethene/Nafion/silica composite membrane with high performance for vanadium redox flow battery [J]. Journal of Power Sources, 2014, 272:113-120.
[34] Chen D Y, Hickner M A, Agar E, et al. Optimized anion exchange membranes for vanadium redox flow batteries [J]. ACS Applied Materials &Interfaces, 2013, 5(15): 7559-7566.
[35] Cui Z L, Drioli E, Lee Y M. Recent progress in fluoropolymers for membranes [J]. Progress in Polymer Science, 2014, 39(1): 164-198.
[36] Hsu W Y, Gierke T D. Ion transport and clustering in Nafion perfluorinated membranes [J]. Journal of Membrane Science, 1983, 13(3): 307-326.
[37] Cui Z M, Xing W, Liu C P, et al. Chitosan/heteropolyacid composite membranes for direct methanol fuel cell [J]. Journal of Power Sources, 2009, 188(1): 24-29.
[38] Shakeri S E, Ghaffarian S R, Tohidian M, et al. Polyelectrolyte Nanocomposite Membranes, Based on Chitosan-phosphotungstic Acid Complex and Montmorillonite for Fuel Cells Applications [J]. Journal of Macromolecular Science, Part B, 2013, 52(9): 1226-1241.
[39] Jae D J, Seung Y K. Ionic cluster size distributions of swollen Nafion/sulfated beta-cyclodextrin membranes characterized by nuclear magnetic resonance cryoporometry [J]. Journal of Physical Chemistry B, 2007, 111(32): 9437-9443.
[40] Zhang B G, Zhang E L, Wang G S, et al. Poly (phenyl sulfone) anion exchange membranes with pyridinium groups for vanadium redox flow battery applications [J]. Journal of Power Sources, 2015, 282:328-334.
[41] Mai Z S, Zhang H M, Zhang H Z, et al. Anion-Conductive Membranes with Ultralow Vanadium Permeability and Excellent Performance in Vanadium Flow Batteries [J]. ChemsusChem, 2013, 6(2): 328-335.
[42] Xu W X, Zhao Y Y, Yuan Z Z, et al. Highly Stable Anion Exchange Membranes with Internal Cross-Linking Networks [J]. Advanced Functional Materials, 2015, 25(17): 2583-2589.

